“Advancing Lung Health in North Carolina”

Roy Pleasants, PharmD – Message from the President
The North Carolina Thoracic Society has completed its full first year since re-forming as a state chapter of the American Thoracic Society with officers – myself, Jason Thomason, MD (Vice President, Salem Chest), Alex Sy, MD (Sec-Treasurer, WFU), and Christina Bellinger, MD (Council Chapter Representative, WFU). An important goal of the Society is to engage both practitioners and researchers – in addition, our bylaws stipulate that officers should include both academic based and private practice based individuals. Jason Thomason recently assimilated a list of private practices in the state and mailed personal invitations to promote membership and our annual meeting to engage colleagues throughout NC. There were approximately 275 private pulmonologists in the state, and nearly 500 pulmonologists if including academic and other work settings as agencies and pharmaceutical companies.
There were some key areas of achievement that should be acknowledged – first, largely through the efforts of Christina Bellinger, MD and Chad Kloefkorn, MD (WFU Pulmonary Fellow), the website for the Society was launched (www.ncthoracic.org) earlier this year. While under construction, there are a number useful tools currently on the site such as inhaler teaching videos and listing of practices. Sections that under construction are: a) an interactive map of private and academic pulmonary practices in the state, b) a practitioner friendly smoking cessation tool (e.g. local and state smoking cessation programs, insurance plan coverage for smoking cessation meds), c) subspecialty practices, and d) clinical research sites.
The Society held its 1st annual meeting last year at Wake Forest University and the next meeting is scheduled Saturday December 8 at UNC CH Friday Center. We have held this meeting jointly with the bronchoscopy course offered to pulmonary fellows in the state. For this year’s annual meeting (rescheduled due to hurricane), we have included a number of interactive workshops as well as activities for fellows as presence of recruiters from NC practices and a workshop on career and job negotiations. Some world-class speakers, mostly from NC, will also present at the morning session.
Two disease areas that we focused on were the two leading cause of respiratory deaths in the state: for COPD we held a successful COPD Readmissions Summit and for lung cancer we are developing a 1 hour CME targeting primary care – with a focus on screening and early diagnosis – for launch early next year at multiple sites in the state.
It was my honor and a great opportunity to help get the NC Thoracic Society underway– I look forward to remaining active in the Society and helping advance lung health in the state.

UNC Researchers Discover Harmful Effects of E-Cigarette Flavoring on Lung Health (Ilona Jaspers, PhD)
Few consumer products have evolved as an environmental health concern as rapidly as e-cigarettes. Introduced into the US just over a decade ago, the use of e-cigarettes, also known as vaping, has skyrocketed in recent years with the development of sleek devices, like JUUL, that utilize flavored e-liquids to deliver nicotine. These e-liquids contain a variety of chemicals like formaldehyde and benzene that can be harmful to respiratory health. Recent research is showing that we can now add flavorings to the list of harmful ingredients in e-liquids with the discovery that cinnamaldehyde, the chemical responsible for cinnamon’s aroma and flavor, impairs the function of cilia in the lungs. At the American Thoracic Society’s international conference in May, Drs. Ilona Jaspers and Phillip Clapp, researchers in the UNC School of Medicine’s program in Toxicology and Environmental Medicine, shared their findings that even one single exposure to cinnamon containing e-liquids and e-liquid vapors resulted in impairment of cilia. Thus, exposure to cinnamaldehyde during vaping could increase the lungs’ susceptibility to respiratory infections. This discovery underscores the need to communicate that using flavored e-liquids in e-cigarettes brings health risks beyond those associated with nicotine use.
Ilona Jaspers, PhD, is the director of the UNC Curriculum on Toxicology & Environmental Medicine, deputy director of the UNC Center for Environmental Medicine, Asthma and Lung Biology, and research director for cardiopulmonary disease in the UNC Center for Environmental Health and Susceptibility. Phillip Clapp conducted this research as a doctoral student in the Jaspers lab and recently completed his PhD.

NC Thoracic Society’s 30-day COPD Hospital Readmission Summit
WakeMed on August 31, 2018
Vikas Pathak, MD — Chair of the Practice and Education Committee and a pulmonologist at WakeMed with faculty appointments at UNC CH and Campbell University — hosted the NC Thoracic Society’s COPD readmissions conference at Wake Med in Raleigh. Since 2015, CMS penalties for excess COPD readmissions within 30 days after hospital discharge has prompted hospitals to take steps to decrease all-cause readmissions for these patients. A new type of provider called COPD Navigator (usually RTs) has emerged in some hospitals to help target these patients. The half-day program brought together approximately 30 hospitals and all major health systems across the state. Six different health system models for COPD readmissions were presented– remarkably, there were many similarities and yet numerous differences among these hospitals (Wake Health, Duke Health, UNC CH, Cone Health, Mission Health, and FirstHealth of the Carolinas). Some of the key messages were recognizing the need for patients to be more engaged in self-care, use of mobile EMTs for home-based interventions, ‘meds to bed” programs, optimizing drug therapies, ways to optimize management in primary care, and increased need for spirometry even during exacerbations if not previously performed. Slide presentations form the Summit are posted on the NC Society website (www.ncthoracic.org).
NC Thoracic Society’s Annual Meeting
December 8 UNC Friday Center
The annual and business meetings will be held at the UNC Friday Center in Chapel Hill on Friday December 7 and Saturday December 8. The business meeting will be held Friday afternoon at 5 PM, followed by a reception and dinner open to interested persons – location to be announced.
The Annual meeting– CME provided–begins the next morning and includes some great speakers and topics of particular interest to practicing pulmonologists. The keynote speaker, David Schwartzstein, MD from Harvard University, will discuss diagnostic uncertainty from the physician and patient perspectives. Other morning topics include an update on sleep disorders, biologics in obstructive lung disease, chronic bronchitis, and presentations by pulmonary fellows from each of the NC training programs. We are also offering some opportunities for fellows including meeting with potential employers from health systems in NC and a workshop on career development and seeking employment. In the afternoon, we have a number of concurrent workshops including cases in ILD and COPD, inhalational therapies, indwelling pleural catheters, high-flow oxygen, and EBUS. There is a $50 fee for the meeting that could also pay for annual membership in NC TS. The annual meeting is free for learners and retired persons. For additional details, visit the NC Thoracic Society’s website (www.ncthoracic.org).

Literature Review – Lung Cancer – (Michael Pritchett, DO, MPH – FirstHealth of the Carolinas)
Assessment of Plasma Proteomics Biomarker’s Ability to Distinguish Benign from Malignant Lung Nodules; Results of the PANOPTIC (Pulmonary Nodule Plasma Proteomic Classifier) Trial Silvestri, G, et al. CHEST 2018; 154(3):491-500 DOI: https://doi.org/10.1016/j.chest.2018.02.012
This was a prospective multicenter trial of 685 patients with lung nodules that measured between 8-30mm. The test uses mass spectrometry to measure the levels of two plasma proteins, LG3BP and C163A. Results were integrated with a clinical risk prediction model to identify nodules that were more likely to be benign.
Among a subgroup of 178 patients with a clinician assessed pretest probability of cancer that was < 50%, the prevalence of cancer was 16%. The novel proteomic test demonstrated a sensitivity of 97%, a specificity of 44% and a negative predictive value of 98% in distinguishing benign from malignant nodules.
This novel classifier performed better than PET scan, validated lung nodule risk modules and physician cancer probability estimates (p < .001).
The authors postulate that if this new proteomic classifier was used to direct care that 40% fewer procedures would be performed on benign nodules. They also point out that approximately 3% of malignant nodules would be misclassified using this test.
Perspective: In the National Lung Screening Trial, 24% of high-risk patients were found to have a concerning pulmonary nodule. Only 4% of those nodules were ultimately determined to be malignant. Current management of nodules involves utilizing pretest probability for malignancy. Many of the identified nodules fall into a low to moderate risk category (0-65% probability of malignancy) where further diagnostic testing may be necessary. Use of a plasma biomarker with excellent sensitivity and negative predictive value for malignancy could potentially avoid unnecessary invasive procedures which come with risks and significant cost. The commercial version of this test is referred to as BDX-XL2 and has recently been acquired by Biodesix, a leader in liquid biopsy molecular testing and proteomics for lung cancer. Biodesix has already designed a multicenter observational registry (ORACLE) to evaluate the performance of the test in patients with a pre-test risk of cancer that is 50% or less. There are 3 sites in North Carolina participating in this registry; East Carolina University, Salem Chest and Pinehurst Medical Clinic.
Cone-Beam CT with Augmented Fluoroscopy Combined with Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules Journal of Bronchology and Interventional Pulmonology, September 2018 – Released Online, Ahead of Print . Pritchett, MA, et al.
This is the first study using cone-beam CT (CBCT)combined with electromagnetic navigation bronchoscopy (ENB) for the diagnosis of peripheral pulmonary nodules. This is a retrospective review of 93 lesions biopsied in 75 consecutive patients (15 patients with multiple lesions including 10 patients with bilateral lesions). Median lesion size was only 16mm. The diagnostic yield by lesion was 83.7%. The minimum and maximum sensitivity for malignancy was 91.3% and 95.5% respectively. The pneumothorax rate was 4%. The majority of patients were diagnosed in stage I or II (79%).
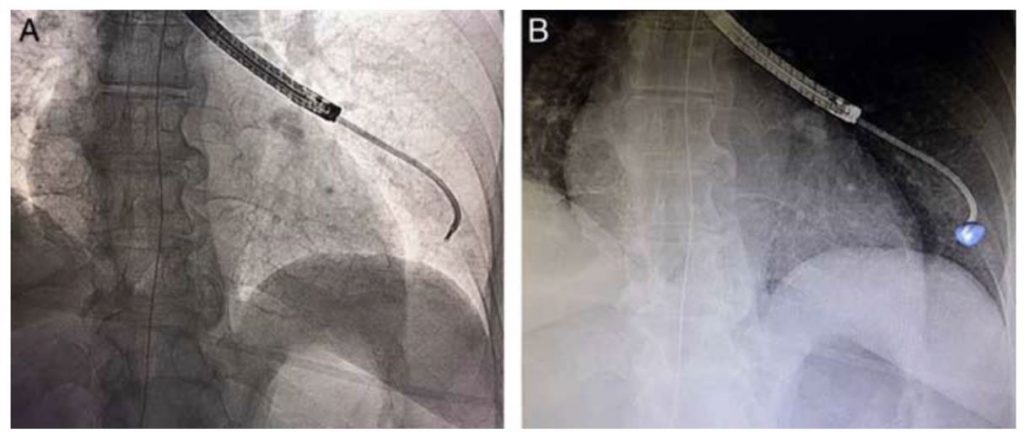
Comparison of standard fluoroscopy (A) with augmented fluoroscopy (B) for a fluoroscopically invisible lesion. The blue volume was segmented from the intra-procedural cone-beam CT data and integrated into the live fluoroscopy image during bronchoscopy.
Perspective: Modern hybrid ORs now offer the ability to acquire cone-beam CT data in addition to standard fluoroscopy. This study highlights the feasibility and effectiveness of CBCT with augmented fluoroscopy during ENB. This combination significantly improved the diagnostic yield compared to ENB alone (as low as 38.5% in the recent large multicenter AQuIRE Registry). The diagnostic yield remained quite high even in lesions 10mm or less (84.2%). This procedure may have the ability to combine the high yield of CT-guided needle biopsy with the safety profile of guided-bronchoscopy. Additionally, biopsy by this method allows for mediastinal staging with EBUS in a single diagnostic procedure. The importance of this technology is already visible based on the fact that CBCT combined with guided-bronchoscopy has already been used for the first-in-human cases of bronchoscopic microwave ablation of lung tumors.

Lung Cancer Screening Registry –
(M.Patricia Rivera, MD)
The North Carolina Lung Screening Registry, is a 5-year (2017-2021) National Institute of Health funded research project working to develop evidence on the utility of low-dose computed tomography (LDCT) to reduce lung cancer mortality that may lead to significant improvements in public health. M. Patricia Rivera, MD, UNC Chapel Hill, Professor, Division of Pulmonary Diseases and Critical Care is a Co-Investigator for this project, providing expertise in the development and conduct of this registry. Dr Rivera is Director, Multidisciplinary Thoracic Oncology Program at UNC CH.
Our long-term goal is to examine the delivery, quality, and outcomes of lung cancer screening in North Carolina by utilizing prospective population-based lung cancer screening data that captures patient, radiologist, and pathology information to inform clinical practice. The objective of this project is to evaluate the extent to which disparities in intermediate lung cancer screening outcomes exist.
We are partnering with communities and researchers across the state to collect data on patients screened for lung cancer from multiple imaging sites in North Carolina. Through this approach, we will build real world evidence on lung cancer screening and outcomes in diverse populations. If you are interested in collaborating on this project, please contact us at 919-966-6160 or Caitlin_bearden@med.unc.edu.
Membership – North Carolina Thoracic Society
The North Carolina Thoracic Society, a state chapter of the American Thoracic Society, would like to engage you to join the society. NCTS members do not have to be members of ATS. We are hopeful to represent lung health professionals in the state, working to engage those in a variety of settings. We plan to facilitate various education activities in the state including holding our annual meeting at different locations. It $50 per year, that also covers the registration cost for our annual CME meeting if you choose to attend– retired persons and learners are free. To join you can go to www.ncthoracic.org or visit the ATS website. https://www.thoracic.org/members/chapters/thoracic-society-chapters/north-carolina/
