
Jason W.W. Thomason, M.D. – Message from the President
From all of us at the NC Thoracic Society, we hope that your summer has been relaxing and rewarding thus far. Each year it seems there is less of a lull in our critical care workload, but hopefully this is still a time of year we can all recharge a bit. Spending quality time with family and friends away from the stresses of Pulm/CC/sleep medicine is essential to our resilience and both our physical and mental health.
One of our primary goals at NCTS is to engage all of us across the state – both in academic and community based settings – so that our sense of community also acts as a very real buffer against burnout. We have numerous upcoming events with which we hope to connect with you and continue to build this network of local healthcare professionals. Please take a few minutes to review the announcements listed below (and/or go to our website for more detailed information) and read some best practices on sleep medicine and digital inhalers.
Warmest regards,
Jason
NCTS Events and Announcements
NC Thoracic Society’s lecture series; “Lung Cancer update for Primary Care”*
Intended to better inform primary care providers about the screening, diagnosis, and treatment for lung cancer. Lung cancer is the leading respiratory-related cause of death in North Carolina and is priority of the Society.
Aug 13; Rowan Medical Center, Salisbury – Christina Bellinger, MD (Wake Forest University)
Sept 17; Forsyth Medical Center, Winston Salem – Mark Bowling, MD (East Carolina University)
Oct 10; Brunswick Medical Center, Bolivia, NC – Michel Pritchett, DO (FirstHealth Carolina)
*Contact for programs kagaydos@novanthealth.org
NC Thoracic Society’s Research Workshop*
Saturday, Sept 21, 2019

Blockade Runner Beach Resort, Wrightsville beach, NC
No charge, workshop is designed to better inform clinical researchers about implementing and conducting clinical trials in different settings. A variety of perspectives will be shared.
*Contact for programs ncthoracicsociety@gmail.com
North Carolina Thoracic Society’s Annual Educational Conference*
Saturday, Nov 16, 2019

The Speedway Club at Charlotte Motor Speedway, 5555 Concord Parkway South, Concord NC.
Topics include critical care, mechanical ventilation, lung cancer, sarcoidosis, pulmonary hypertension, and fellows’ presentations among others. (Host: Jaspal, Singh, MD Atrium Health)
*Contact for programs ncthoracicsociety@gmail.com, https://www.ncthoracic.org
State of North Carolina Respiratory Health Survey
The North Carolina Department of Public Health administers a CDC telephone health survey each year to about 10,000 NC adults called the “Behavioral Risk Factor Surveillance System”. In 2018, the state included a set of questions called the Respiratory Health Module. This set of questions, combined with numerous others in the survey, will provide the most extensive perspective of lung health ever undertaken in North Carolina. The Society will be obtaining, analyzing, and generating a Lung Health Report for the state to post on the website as well as use and distribute for advocacy.
Website Updates
The Society’s website (https://www.ncthoracic.org) was recently updated to include interactive maps of North Carolina that show contact information for adult and pediatric pulmonary practices in the state. Also, there is a new interactive map showing clinical research sites for pulmonary medicine in the state. To provide additional input for these maps, email ncthoracicsociety@gmail.com
New NCTS Secretary/Treasurer announced: James Davidson, MD
All of us greatly appreciate Dr. James Davidson of Asheville Pulmonary and Critical Care Associates accepting the nomination from the NCTS executive committee to fill a vacancy with the departure of Vikas Pathak, MD. We wish Dr Vikas Pathak all the best at his new position in Newport News, VA!
Call for Nominations
We are seeking nominees for NCTS Secretary-Treasurer (2020), NCTS Council Chapter Representative (2020-2022), and NCTS Clinician of the Year award.
White Coat Wednesdays
The North Carolina Medical Society helps facilitate White Coat Wednesdays during sessions of the General Assembly. Great opportunity to meet with NC legislators to advocate for lung health. www.ncmedsoc.org/advocacy/legislative-issues/white-coat-wednesdays
Membership in NCTS
If you are already an NCTS member, we thank you for your support and hope that you will share this newsletter with a colleague! If you are considering becoming a member for only $50/year – which also covers the cost of attending the annual meeting and the associated Category I CME – then please do one of the following:
- check out our website www.NCTHORACIC.org
- simply go to this direct link: https://www.thoracic.org/members/chapters/thoracic-society-chapters/north-carolina, or
- visit our facebook page https://www.facebook.com/NCthoracicsociety/

Best Practices
Perioperative OSA Screening and Treatment: Is it really an option?
Andrew M. Namen, MD, FCCP, FAASM and Daniel J Forest, MD (Section on Pulmonary, Critical Care, Allergy and Immunologic Diseases, Wake Forest University School of Medicine)
Obstructive sleep apnea (OSA) is a major cause of morbidity and mortality in the U.S.1 Although there continues to be improvement in the screening and diagnosis of this disease, many patients remain undiagnosed which carries increase morbidity and medical care cost.2,3 Recent national provider survey data demonstrate that a paucity of preoperative visits have OSA as an associated diagnosis.4 With the continued rise in obesity, the prevalence and burden of OSA will likely increase and the gap between undiagnosed and diagnosed OSA will correspondingly widen..5 Several sleep questionnaires have been used in the preoperative arena with the goal of identifying patients at risk for OSA prior to elective procedures.6,7 Previous studies demonstrate that patients with OSA have an increase in postoperative atelectasis, pneumonia and oxygen desaturations and patients on CPAP have reduced number of these events.8 Recent studies have demonstrated that unrecognized patients at risk for OSA have an increase all-cause mortality and medical emergency team activation during hospitalization whether peri-operatively or on a medicine ward service.9, 10 Given these concerns best practice include identifying patient at risk and those with known diagnosis. Patients with known OSA should be encouraged to use their home therapy during the perioperative period. High risk patients without confirmed diagnosis should be encouraged to be tested and possibly treated prior to surgery if situation allows. Alternatively using auto-pap during the peri-operative period seems reasonable in high risk patients without a confirmed sleep study who require time sensitive surgery.
1. Young T, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071-8.
2. Peppard PE, et al., Increased prevalence of sleep-disordered breathing in adults. American journal of Epidemiology. 2013;177(9):1006-14.
3. AASM. “Economic Impact of Obstructive Sleep Apnea.” Retrieved 6/27/2019, from http://www.aasmnet.org/sleep-apnea-economic-impact.aspx.
4. Namen AM, Forest DJ, et al. Physicians report sleep apnea infrequently in older and older vulnerable adults. J Am Geriatr Soc. 2017 Sep;65(9):2023-2028.
5. Namen AM, Haponik EF et al. Recognition of sleep apnea Is Increasing. analysis of trends in two large, representative databases of outpatient practice. Ann Am Thorac Soc. 2016 Nov;13(11):2027-2034
6. Namen AM, Forest DJ, et al., Preoperative sleep qestionnaires identify medical emergency team activation in older adults. J Am Med Dir Assoc. 2019 Jun 11. pii: S1525-8610(19)30392-5. doi: 10.1016/j.jamda.2019.04.024
7. Chung F, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822-30.
8. Kaw R, et al., Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436-41
9. Chan MTV, et al., Postoperative Vascular Complications in Unrecognized Obstructive Sleep Apnea (POSA) Study Investigators. JAMA. 2019 May 14;321(18):1788-179p
10. Pickens AW, Forest DJ et al., Identifying risk of sleep apnea and major hospital events in an older inpatient opulation. J Am Geriatr Soc. 2018 Sep;66(9):1847-1848. doi: 10.1111/jgs.15429.

Digital Inhalers – An Advance for Monitoring Medication Use and Disease Management
Roy A. Pleasants, PharmD (Executive Director, North Carolina Thoracic Society; UNC Chapel Hill Division of Pulmonary Medicine and Critical Care; and Durham VA Medical Center)
Digital inhaler devices are now available that utilize electronic sensors that transmit usage data via Bluetooth technology to smartphones and ultimately to a server that can be accessed remotely and real-time by providers and patients. Among other functions, these devices provide audible alerts to remind patients to use their maintenance inhalers, while documenting adherence. For rescue inhalers, use is recorded and patients can be alerted by their smartphone to seek medical help based on frequency of use and symptoms and other health data the patient provides. Other features of these apps are essentially disease management programs, currently available for asthma and COPD. In a study of the Diskus® with a digital sensor, the majority of 244 COPD patients discharged from the hospital were found to be frequently noncompliant and often used their inhaler incorrectly – despite getting a free inhaler and education!1 If only we really knew!!
Two digital inhaler sensor devices are FDA approved – Propeller Health (2016) and the Digihaler® (Proair® and Airduo®) by Teva (commercially available 2020). The Propeller Health sensors (below) are attached directly to existing inhalers including the MDI, Respimat®, Diskus®, and Ellipta®. Some inhalers, like Symbicort® MDI, are not compatible. Whereas Propeller devices are “add-ons”, the Digihaler®(below) has a built-in sensor that transmits usage data digitally; and unlike other marketed devices, records inspiratory flow. There is increasing evidence showing that some patients, particularly COPD, are not achieving adequate inspiratory effort for dry powder inhalers. This device lets the patient know if they can achieve a proper inhalation and the provider can access this information. Digihaler® also has the potential to monitor lung function by changes in inspiratory flow that occur with worsening respiratory status in obstructive lung diseases.
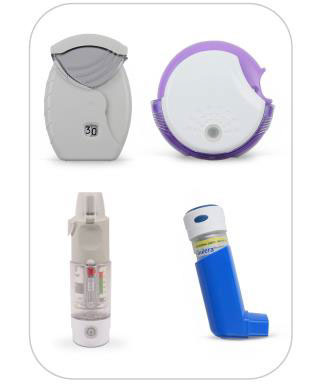

Clinical data show that the Propeller devices can improve outcomes in COPD and asthma through improving compliance and alerting patients to seek medical help.2 A recent study of the Digihaler® showed that imminent asthma exacerbation could be detected by increased albuterol use and changes in inspiratory flow measures (PIFR, Insp vol).3
Currently, use of these devices in NC is limited. Typically, healthcare systems and payers contract with Propeller to provide digital devices directly to patients. The process for the Digihaler is not yet defined. Of note for providers, CMS posted 2019 billing codes for remote patient monitoring technology that may provide a mechanism to bill for services related to digital inhalers (99453, 99454,99457). There are a number of issues that need addressing including insurance coverage, medicolegal liability and determining patient types most likely to benefit. I think some targeted uses include documenting inhaler compliance in a severe asthmatic being considered for a biologic or a COPD patient post-hospitalization. (Dr Pleasants discloses COI with Teva for consulting and research)
1. Sulaiman I, Cushan B, Greeen G et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:1333–134
2. Chen J, Jones-Ford S, Goeldi S et al. Feasibility and clinical impact of deploying a digital health intervention on a Medicare population with asthma or chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med 2017;195:A1722
3. Safioti G, Granovsky L, Li T et al. A predictive model for clinical asthma exacerbations using albuterol eMDPI (ProAir Digihaler): a 12- week, open-label study. Am J Respir Crit Care Med 2019;199:A7307
Officers – North Carolina Thoracic Society
President: Jason W.W. Thomason, MD, FCCP, D-ABSM; Winston-Salem. Salem Chest Specialists (jwwt@triad.rr.com)
Vice President: Alex Sy, MD, MBA, FCCP, FACP, FAASM; Winston Salem. Wake Forest Division of Pulmonary Critical Care and Sleep Medicine, (asy@wakehealth.edu)
Secretary/Treasurer: James Davidson, MD, FCCP; Asheville. Asheville Pulmonary and Critical Care Associates (jamesjasdavidson@gmail.com)
Chapter Councilor: Christina Bellinger, MD; Winston-Salem. Wake Forest Division of Pulmonary Critical Care and Sleep Medicine (Cbelling@wakehealth.edu)
Executive Director – Roy Pleasants, PharmD ncthoracicsociety@gmail.com
