“Advancing Lung Health in North Carolina”
James Davidson, M.D. – Message from the President
Hello (again) and greetings from the North Carolina Thoracic Society (NCTS), your state chapter of the American Thoracic Society. As we begin to reflect on the past year and look forward into our post-pandemic landscape, I want to reiterate the NCTS’s dedication to building relationships between all of our outstanding North Carolina practices both academic and private. As pandemic restrictions lessen we will again look to engage with our private practice and rural community practitioners though in-person and virtual educational meetings and outreach talks. We currently have our annual educational conference scheduled for Saturday, September 11th in beautiful Asheville, NC. We are also re-starting our Meet the Professor with an ILD presentation by Stephen Nathan, MD at Cone Health inJune that is being hosted by Praveen Mannam, MD (NCTS Sec Treasurer) and Murali Ramaswamy, MD. We plan to make this daytime program virtually available to other practices in the state.
To continue and build upon our success, we hope you consider joining our community of outstanding healthcare providers and thought leaders. NCTS membership is just $50 annually. Go to www.NCTHORACIC.orgto review up to date content and find a membership application. You can also find and like us on Facebook.
Sincerely,
James Davidson
Chapter President 2020-2021 2
NCTS Events and Announcements
2021 North Carolina Thoracic Society Annual Educational Conference
Our annual conference is set for Saturday September 11, 2021 in Asheville at the Biltmore Doubletree Hotel.
We plan to have a live program, and will provide virtual access for those interested in remote attendance. Continuing medical education will be provided through Novant Health. Speakers include M. Patricia Rivera MD, UNC-CH (ATS Vice-President) discussing new cancer screening guidelines; Craig Rackley, MD (Duke University) and Jesse Madden, MD (Mission St Joseph) discussing ECMO; Jesse Turner MD (Wake Forest and Physician Philosopher) discussing physician burnout, and Chad Marion, MD, PhD (Wake Forest University) discussing management of non-CF bronchiectasis, followed by clinical and research presentations by pulmonary fellows from the state’s training programs.
Please consider attending and meet some of your peers across the state and enjoy the Asheville area with friends and family. We also will have a Friday evening dinner and educational program. Details on registration will be provided in a SaveTheDate meeting announcement. Of course renewing your NCTS membership allows you free attendance.
Membership in NCTS
If you are already an NCTS member, we thank you for your support and hope that you will share this newsletter with a colleague! If you are considering becoming a member for only $50/year – which also covers the cost of attending the annual meeting and the associated Category I CME – then please do one of the following:
1. simply go to this direct link: https://www.thoracic.org/members/chapters/thoracicsociety-chapters/north-carolina, or
2. visit our facebook page https://www.facebook.com/NCthoracicsociety/
FELLOWS CORNER (new!!)
Jennifer Krall, MD (PGY6 Fellow in Pulmonary and
Critical Care Medicine)
A Reflection on Fellowship Training in a Pandemic
On March 3, 2020, North Carolina reported its first case of Coronavirus Disease 2019 (COVID-19). Nine days later, the World Health Organization declared the COVID-19 outbreak a global pandemic. Many 3
changes quickly occurred in our personal and professional lives, several of which are still in place today. Numerous aspects of fellowship training have been affected, as well, in both negative and positive ways. The challenges of this pandemic have provided lessons in adaptation that we carry with us as we move forward. Early on, concerns for safety and preservation of resources accompanied the arrival of COVID-19 to our state. Much of what we had known about the virus was from observing what was happening in other countries as well as the early epicenters in the United States. We had to protect our workforce, and fellows that were not already scheduled to work in the ICU or on the consult service were asked to work from home. Non-urgent outpatient clinic visits were postponed. Elective procedures were rescheduled, which is difficult for trainees in a procedure heavy training path. These changes were necessary to prepare us for the unknowns of the pandemic. Outside of our usual clinical duties, the environment had to be modified as well to protect everyone from the virus. The fellows’ office, which also served as a shared area for congregation and commiseration, became vacant. It became apparent how much we relied on this spontaneous conversation throughout the workday, whether it was to help each other work through a difficult problem or just a lighthearted discussion on recent events. It was harder to maintain research and scholarly activity, another pillar of fellowship training, with labs closed and clinical trials on pause. In short, the pandemic placed many obstacles in many core aspects of training. As our division made plans to care for an influx of COVID-19 patients, many changes occurred to meet and overcome this challenge. Our fellows adopted a new messaging application and quickly became engaged in conversations. These conversations varied from what was going on in the hospital, comedic gifs, and pictures from new hobbies we were picking up such as sourdough bread baking and gardening. Our chief fellow at that the time shared a video of himself making aloo gobi. The conversation kept this time of uncertainty livelier. Video conferencing quickly became a valuable resource. We established weekly check-ins with our program leadership. This was a time for us to share our worries and trepidations but also allowed us to see each other, sometimes pets and other family members included. Video conferencing also became a venue for education. We restarted our educational conferences which has taught us new and interesting uses of this technology. There was less spontaneous commentary and questions initially, but soon enough we learned how to have those conversations using the chat feature. Before long, division attendings and fellows were bringing their flair to the meetings with interesting hats and backgrounds. Further, national conferences have been able to restart allowing people to attend without the costs of travel. In some ways, it was easier to meet with and collaborate with people from other states and institutions. Nationally, there were also major changes to fellowship recruitment. Our program used multiple platforms to accomplish this including a refresh to our fellowship website, new recruitment videos featuring attendings and fellows, and video conferencing to interview potential candidates. We have successfully recruited a new class of fellows and look forward to welcoming them in person. In many ways, our response to the challenges of the pandemic has made us closer as a fellowship and certainly more innovative. We have gained many lessons from this pandemic. Video conferencing will continue to be an option that allows attendance at conferences that might have otherwise been difficult. It has the potential to allow mentorship relationships to develop, perhaps across institutions. Additionally, recruitment of fellowship candidates will likely continue to evolve allowing better access to interviews and perhaps a financial savings from the burden of travel. Finally, we will have a better appreciation of the day-to-day interactions we have with each other, family and friends. Striking a balance in our commitment to our fellowship training, patient care, and personal health will be more accessible with the lessons we have gained from this pandemic.
Special thanks to Dr. Matt Miles for his input on this article and fellowship leadership.
For more on fellowship learning during the pandemic, check out this webinar from CHEST: https://vimeo.com/452704201https://vimeo.com/452704201
https://vimeo.com/452704201 4
DISEASE UPDATE
Chad Marion, MD, PhD Assistant Professor, Section of Pulmonary, Critical Care, Allergy and Immunologic Diseases
Bronchiectasis in Adults: A Brief Overview
Bronchiectasis (BE) is the permanent dilation of the airways and in the absence of cystic fibrosis (non-CF BE). The prevalence of non-CF BE increases with age and has an estimated prevalence of over 800/100,000 in patients ages 75 years or older. BE results from a pathogenic cycle of impaired mucociliary clearance, infection, inflammation, and airflow obstruction(1).
Computer tomography of the chest is the diagnostic test of choice to confirm BE, while the radiographic pattern and distribution provides diagnostic clues as to potential etiologies based on diffuse, bilateral with upper or lower lobar predilection, or focal disease. Focal bronchiectasis etiologies include post-infectious (tuberculous and nontuberculous mycobacterium (NTM) infections, pertussis), congenital bronchial atresia, endobronchial tumors, foreign bodies, and extrinsic airway compression and usually warrants bronchoscopic evaluation. The diagnostic workup for diffuse or bilateral BE initially should test for humoral immune deficiency and allergic bronchopulmonary aspergillosis (ABPA, serum IgE), and airway cultures, including acid-fast staining and culture. Subsequent testing should include sweat chloride sweat testing and CFTR genetic testing. Depending on the clinical manifestations, additional diagnostic tests include nasal epithelial ciliary biopsy for motion or electron microscopy, nasal nitric oxide concentrations, primary ciliary dyskinesia gene pathway testing, alpha-1 antitrypsin concentrations, and evaluation for systemic autoimmune diseases associated with bronchiectasis, such as colitis and rheumatoid arthritis. Despite rigorous diagnostic testing, approximately half of patients will have idiopathic disease(2, 3).
Treatments should be specific to the underlying etiology if identified. In all cases of bronchiectasis, supportive management to facilitate airway clearance is critical for long-term management and the acute management of exacerbations. The addition of inhaled beta2-adrenergic receptor agonists and nebulized saline compliments the first-line supportive management with regular chest physiotherapy. While there is data on the use of formoterol and budesonide, most guidelines recommend reserving the use of inhaled corticosteroids for patients with co-morbid asthma(4, 5). Additional adjunctive therapies include chronic macrolide therapy and suppressive inhaled antibiotics(6, 7).
References
1. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasiamong US adults in 2013. Chron Respir Dis 2017; 14: 377-384.
2. Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, Johnson M, Eden E, Griffith D, Knowles M, Metersky M, Salathe M, Thomashow B, Tino G, Turino G, Carretta B, Daley CL. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017; 151: 982-992.
3. Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, Polverino E, Van de Kerkhove C, Rutherford R, Davison J, Rosales E, Pesci A, Restrepo MI, Torres A, Aliberti S. Etiology of Non-Cystic Fibrosis Bronchiectasis in Adults and Its Correlation to Disease Severity. Ann Am Thorac Soc 2015; 12: 1764-1770.
4. Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn JS, Floto RA, Grillo L, Gruffydd-Jones K, Harvey A, Haworth CS, Hiscocks E, Hurst JR, Johnson C, Kelleher WP, Bedi P, Payne K, Saleh H, Screaton NJ, Smith M, Tunney M, Whitters D, Wilson R, Loebinger MR. British Thoracic Society guideline for bronchiectasis in adults. BMJ Open Respir Res 2018; 5: e000348.
5. Visser SK, Bye P, Morgan L. Management of bronchiectasis in adults. Med J Aust 2018; 209: 177-183. 5
6. Kelly C, Chalmers JD, Crossingham I, Relph N, Felix LM, Evans DJ, Milan SJ, Spencer S. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst Rev 2018; 3: Cd012406.
7. Kocurek EG, Jagana R. Noncystic fibrosis bronchiectasis management: opportunities and challenges. Curr Opin Pulm Med 2019; 25: 192-200.
INNOVATIONS IN PULMONARY MEDICINE

Alper Bozkurt, PhD Professor of Electrical and
Computer Engineering at NC State University

Michelle Hernandez, MD Associate Professor
of Pediatrics, UNC School of Medicine

Edgar Lobaton, PhD Associate Professor of Electrical
and Computer Engineering at NC State University
Wearable Hardware and AI Innovations for Asthma and Cough Monitoring
Asthma exacerbations are characterized by bronchoconstriction, mucus production, and inflammatory cell recruitment [1-3]. The autonomous nervous system regulates these physiological responses as well as heart rate and respiratory rate which can be monitored continuously during activities of daily life using wearable sensors. Wearable technology can also measure a host of other physiologic parameters
that may be predictive of an impending asthma exacerbation including heart rate variability, activity levels, galvanic skin response, wheezing, and coughing. In particular, a recent study suggested that nocturnal cough and sleep quality have useful properties as markers for asthma control and may enable early detection of asthma attacks [6].
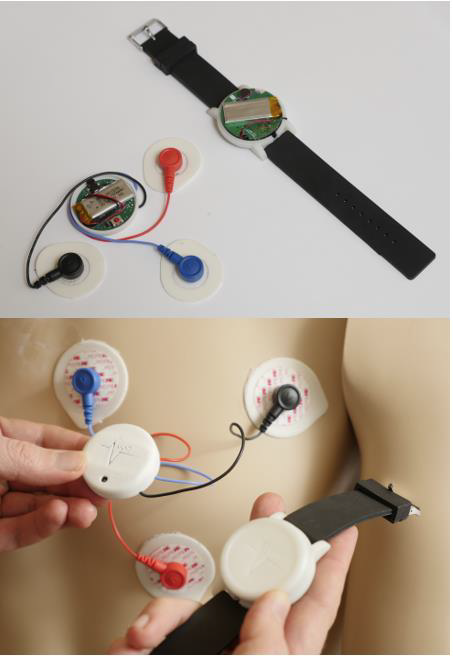
Fig. HET Prototype produced by NCSU ASSIST Center. 6
Cough recordings can also be used for diagnosis of other medical conditions. For example, an Artificial Intelligence (AI) system was trained to identify individuals with COVID-19 from audio recordings of forced coughs and reported over 90% sensitivity and specificity [7]. The data used for this study was composed of recordings of forced coughs of individuals obtained through a web app, and self-report of symptoms and diagnosis of COVID-19. Hence, continuous monitoring of cough using a wearable device has great potential to enable early detection of respiratory ailments and reduce health care costs.
Several commercial-off-the-shelf and research prototype wearable hardware have been used in recent clinical studies combining respiratory measurements, accelerometers, and microphones [8,9,12]. More recently, devices using only accelerometers instead of microphones were introduced by academic research teams [15] and startup companies [14,16] for lower power consumption, lower cost and much smaller form factors. Under the National Science Foundation (NSF) Nanosystems Engineering Research Center for Advanced Self-Powered Systems of Integrated Sensors and Technologies (ASSIST), our collaborative team, comprising members from NC State University and University of North Carolina at Chapel Hill School of Medicine, has been developing key core technologies for achieving a high-performance and multi-functional wearable biomedical sensing system while minimizing power consumption. The ASSIST partnership developed the Health and Environmental Tracker (HET) testbed (see Figure) as a wireless ultra-low power system for correlated sensing of multiple environmental and health parameters [A1, A2]. The developed prototype device includes a wristband and chest patch to monitor various asthma-related physiological parameters. The wristband sensors track wrist motion and heart rate via photoplethysmography. It can also sense changes in the environment including ambient temperature, relative humidity, and outdoor and indoor air pollutants such as ozone and volatile organic compounds. The chest patch sensors are capable of measuring electrocardiography for heart rate, skin impedance for cough and respiration detection, photoplethysmography for heart rate and respiratory rate, chest motion for activity levels, and acoustic signals for wheezing and coughing. In order to handle the large and continuous amount of data being generated by these devices, the system constantly transmits the recordings to a Bluetooth Low Energy enabled peripheral data aggregation device (e.g. a laptop, tablet computer, or smartphone). The HET system received the Best Paper Award in the prestigious IEEE Body Sensor Networks Conference in 2015 and was tested both on animals [A3] and human subjects [A1]. This non-commercial research prototype system is also accessible to ASSIST Center’s research partners and medical collaborators. The current efforts are towards getting this system powered using energy harvested from the user’s body heat and motion to enable a battery-free and wear-and-forget experience for the user.
One of our latest efforts has focused on improving the accuracy of cough detection using machine learning techniques on acoustic signals while minimizing speech recognition to guarantee privacy for users of this technology. Studies have achieved high sensitivity (~ 90%) in controlled clinical settings using standard machine learning techniques [12] and deep learning models [19]. Using a deep learning-based cough detection algorithm, preliminary analysis of our system maintained an average sensitivity above 90%. We are complementing this microphone-based approach for cough detection with other modalities including the use of photoplethysmography and accelerometers signals in the wristband and chest-patch. The accelerometers directly measure the ballistic motion of the chest during coughing, while the motion artefact on the photoplethysmography signal provides a complementary measurement. Combined together, these sensors have a great potential to detect presence, frequency and severity of coughs that can be applied to the diagnosis and management of asthma and other respiratory conditions.
[1] A. J. Stoppelenburg, V. Salimi, M. Hennus, M. Plantinga, R. H. I. Veld, J. Walk, J. Meerding, F. Coenjaerts, L. Bont, M. Boes, and et al., “Local il-17a potentiates early neutrophil recruitment to the respiratory tract during severe rsv infection,” PLoS ONE, vol. 8, no. 10, 2013.
[2] J. Zhu, S. D. Message, Y. Qiu, P. Mallia, T. Kebadze, M. Contoli, C. K. Ward, E. S. Barnathan, M. A. Mascelli, O. M. Kon, and et al., “Airway inammation and illness severity in response to experimental rhinovirus infection in asthma,” Chest, vol. 145, no. 6, p. 12191229, 2014. 7
[3] K. Grnberg, H. H. Smits, M. C. Timmers, E. P. De Klerk, R. J. Dolhain, E. C. Dick, P. S. Hiemstra, and P. J. Sterk, “Experimental rhinovirus 16 infection. effects on cell differentials and soluble markers in sputum in asthmatic subjects,” American Journal of Respiratory and Critical Care Medicine, vol. 156, no. 2, p. 609616, 1997.
[6] P. Tinschert, F. Rassouli, F. Barata, C. Steurer-Stey, E. Fleisch, M. A. Puhan, T. Kowatsch, and M. H. Brutsche, “Nocturnal cough and sleep quality to assess asthma control and predict attacks,” Journal of asthma and allergy, vol. 13, p. 669, 2020.
[7] J. Laguarta, F. Hueto, and B. Subirana, “COVID-19 Artificial Intelligence Diagnosis Using Only Cough Recordings,” IEEE Open Journal of Engineering in Medicine and Biology, vol. 1, pp. 275– 281, 2020.
[8] M. A. Coyle, D. B. Keenan, L. S. Henderson, M. L. Watkins, B. K. Haumann, D. W. Mayleben, and M. G. Wilson, “Evaluation of an ambulatory system for the quantification of cough frequency in patients with chronic obstructive pulmonary disease,” Cough, vol. 1, no. 1, pp. 1–7, 2005.
[9] S. Leconte, G. Liistro, P. Lebecque, and J.-M. Degryse, “The objective assessment of cough frequency: Accuracy of the lr102 device,” Cough, vol. 7, no. 1, pp. 1–8, 2011.
[12] S. S. Birring, T. Fleming, S. Matos, A. A. Raj, D. H. Evans, and I. D. Pavord, “The Leicester Cough Monitor: Preliminary validation of an automated cough detection system in chronic cough,” European Respiratory Journal, vol. 31, no. 5, pp. 1013–1018, May 1, 2008.
[14] M. Sterling, H. Rhee, and M. Bocko, “Automated cough assessment on a mobile platform,” Journal of medical engineering, vol. 2014, 2014.
[15] K. Lee, X. Ni, J. Y. Lee, H. Arafa, J. P. David, S. Xu, R. Avila, M. Irie, J. H. Lee, R. L. Easterlin, et al., “Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch,” Nature biomedical engineering, vol. 4, no. 2, pp. 148–158, 2020.
[16] S. P. Schmidt, Cough detection, analysis, and communication plat- form, US Patent 10,820,832, Nov. 2020.
[19] F. Barata, K. Kipfer, M. Weber, P. Tinschert, E. Fleisch, and T. Kowatsch, “Towards Device-Agnostic Mobile Cough Detection with Convolutional Neural Networks,” in 2019 IEEE International Conference on Healthcare Informatics (ICHI), Jun. 2019, pp. 1–11.
[A1]Dieffenderfer J, Goodell H, Mills S, McKnight M, Yao S, Lin F, Beppler E, Bent B, Lee B, Misra V, Zhu Y, Oralkan O, Strohmaier J, Muth J, Peden D, Bozkurt A. Low-Power Wearable Systems for Continuous Monitoring of Environment and Health for Chronic Respiratory Disease. IEEE J Biomed Health Inform 2016; 20: 1251-1264.
[A2] Misra V, Bozkurt A, Calhoun B, Jackson TN, Jur JS, Lach J, Lee B, Muth J, Oralkan O, Ozturk M, Trolier-McKinstry S, Vashaee D, Wentzloff D, Zhu Y. Flexible Technologies for Self-Powered Wearable Health and Environmental Sensing. P Ieee 2015; 103: 665-681.
[A3]. Brugarolas R LT, Dieffenderfer J, Walker K, Sherman B, Roberts D, Bozkurt A. Wearable Heart Rate Sensor Systems for Wireless Canine Health Monitoring. IEEE Sensors Journal 2016; 16: 3453-3464
Officers – North Carolina Thoracic Society
President: James Jas Davidson, MD, FCCP; Asheville, Asheville Pulmonary and Critical Care Associates jamesjasdavidson@gmail.com
Vice President: Mashael Al-Hegelan, MD; Durham, Assistant Professor, Duke University Division of Pulmonary, Allergy, and Critical Care Medicine
Secretary-Treasurer: Praveen Mannam, MD, Greensboro, Cone Health
Chapter Councilor: M. Brad Drummond, MD, MHS; Chapel Hill, Associate Professor, UNC at Chapel Hill
Division of Pulmonary Diseases and Critical Care Medicine
Executive Director – Roy Pleasants, PharmD ncthoracicsociety@gmail.com